Gain a comprehensive understanding of Hatch-Waxman and BPCIA essentials, a critical competency for legal and business professionals in the biopharmaceutical arena.
Welcome to ACI’s Hatch-Waxman and BPCIA Proficiency Series, a virtual three-week program designed to provide new lawyers and executives for the life sciences industry with a solid foundation for understanding the essentials as well as the intricacies of Hatch-Waxman and BPCIA litigation and regulation.
Everyone who works in the life sciences industry must be well versed in the regulatory components and IP subtleties that play such an integral role in the patenting of its products.
A thorough understanding of Hatch-Waxman and the BPCIA is essential to anyone working in the biopharmaceutical area. This series, presenting virtually on our new state of the art interactive platform, will provide an in-depth review of Hatch-Waxman and the BPCIA as well as other IP basics relative to small molecules and biologics. This program will lay the necessary foundation to understand the dynamics of the applicable patent life cycles for biopharmaceutical products and business development plans.
Take a look at the 2023 Agenda and Speakers
Week One
|
Week Two
|
Week Three
|
Co-Chairs

Li Feng, Ph.D.
Partner
Finnegan

Rachel Pernic Waldron
Partner
RMMS Legal
“A robust and balanced FDA/patent linkage system is critical to incentivizing innovation and facilitating access to lower cost medicine. The USPTO and FDA has recently announced collaboration initiatives to further this goal. I am excited to join industry experts and government representatives to provide this foundational course for anyone who is interested in practicing at the intersections of FDA and patent law.”
“The biopharmaceutical space is ever-changing, and yet a thorough understanding of Hatch-Waxman and the BPCIA—not to mention the interaction between the USPTO and FDA–is essential to anyone working in this area. I am thrilled to be a part of this immersive series, which explores a variety of litigation and regulatory issues, including the interplay between these key agencies. I very much look forward to hearing from industry experts, including skilled advocates from ‘both sides of the v.'”
What you can expect
- 3 weeks of 2x weekly in-depth instruction
- 18 hours of interactive learning for professional development
- Dedicated questions and answer period each day
- 6 class recordings for future reference
- Substantive resource materials for your daily work
- Certificate of completion and CLE credits
Practical Benefits to Attending
- UNDERSTAND the interplay of the PTO and FDA in the patenting of drugs and biologics
- LEARN about the approval process for drugs and biologics and their connection to the patent process
- DEVELOP an in-depth and practical knowledge of Hatch-Waxman protocols, including:
- Differences between NDAs, ANDAs, BLAs, aBLAs
- The Orange Book vs. The Purple Book
- 180 Exclusivity
- Bioequivalency
- The Safe Harbor
- COMPREHEND the framework of Hatch-Waxman Paragraph IV litigation and BPCIA patent dance
- MAKE SENSE of the relationship between patent and non-patent exclusivity
- EXAMINE patent extensions including restorations and adjustments
- NAVIGATE the rules and exceptions of the safe harbor
Who Should Attend
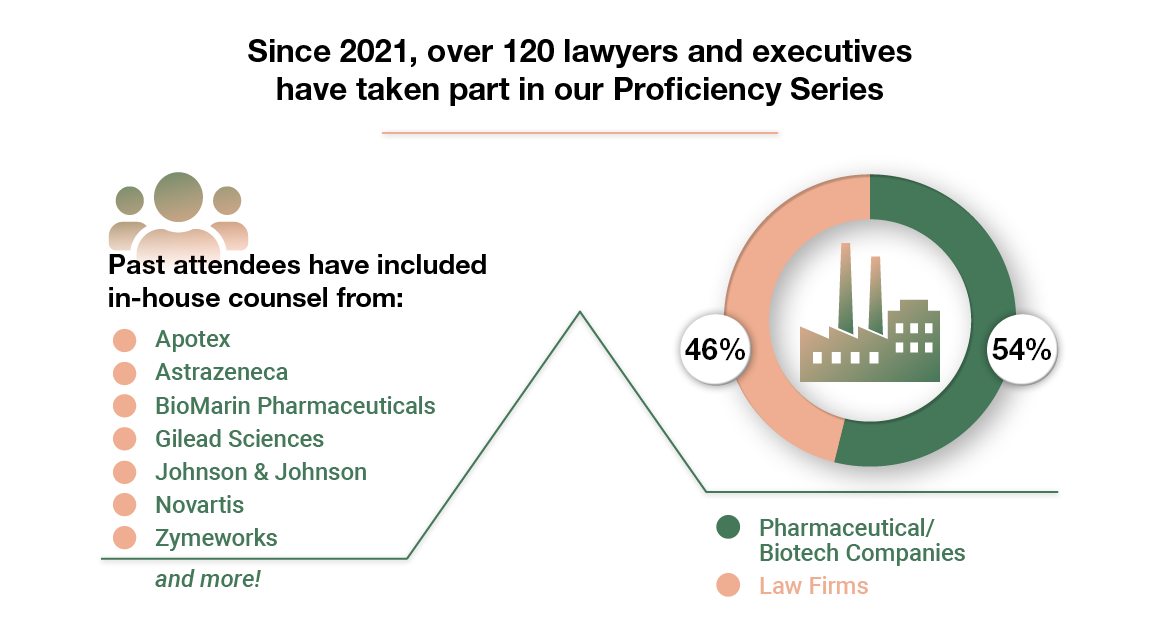
The Hatch-Waxman and BPCIA Essentials Proficiency Series is designed for new Associates and business executives for the life sciences industry.
LAW FIRMS
Attorneys assigned to the following practice groups:
- Life Sciences, Pharmaceuticals, and Biopharmaceuticals
- Intellectual Property and Patents
- Patent and IP Litigation
- Hatch-Waxman and BPCIA litigation
- Patents, Trademark & Copyright
PHARMACEUTICAL COMPANIES
- In-House Counsel
- Business Development Executives
- C-Suite Executives
- Associate Executives
Join Our Mailing List
Receive exclusive discounts, offers and agenda updates directly to your inbox.






