Master Hatch-Waxman and BPCIA Essentials for Legal and Business Professionals in the Biopharma Arena
Unlock a critical skillset tailored for legal professionals in the biopharmaceutical sector. ACI’s Hatch-Waxman and BPCIA Proficiency Series is a virtual, three-week intensive program designed to equip new life sciences lawyers and executives with the expertise needed to navigate Hatch-Waxman and BPCIA litigation and regulation.
In today’s complex biopharmaceutical landscape, understanding the regulatory and IP nuances is essential for securing and defending patents. This series delivers a thorough examination of Hatch-Waxman and the BPCIA, alongside foundational IP insights for both small molecules and biologics, empowering you to strategically manage patent lifecycles and guide business development decisions effectively.
Join us on our state-of-the-art interactive platform to gain the essential knowledge and tools that underpin success in the life sciences legal field.
Program Co-Chairs

Chris Bruno
Partner
McDermott, Will & Emery LLP

Tara Raghavan
Partner
Benesch Friedlander Coplan & Aronoff LLP
Featured Faculty

Rob Vrana
Partner
Young Conaway, Stargatt & Taylor, LLP

Sara Koblitz
Director
Hyman, Phelps, & McNamara PC

Michael Stern
Of Counsel
Covington & Burling LLP

Irena Rozyman
Partner
Orrick, Herrington & Sutcliffe LLP

Andrew Wasson
Partner
Haug Partners
Key Program Modules
Week 1
Regulatory Foundation
- Interplay of the FDA and PTO
- Pre-Commercialization Concerns
- Link between the FDA Approval and the Patent Process
- The Orange Book
Week 2
Hatch-Waxman and BPCIA Framework
- The Hatch-Waxman Landscape
- Paragraph IV Disputes and Litigation
- Biosimilars: BPCIA and aBLA Overview
- Participating in the Patent Dance
- The Purple Book
Week 3
Focus on Bioequivalence, Exclusivity, Extensions, and Exceptions
- Bioequivalence & Interchangeability
- 180-Day Exclusivity
- Non-Patent/ Regulatory Exclusivity
- Exploring the Safe Harbor
- Examining Patent Extensions
Advisory Board
Gain exclusive insights from our esteemed Hatch-Waxman advisory board – trailblazing professionals whose expertise and leadership have been instrumental in shaping this year’s comprehensive program on key industry developments, litigation trends, and emerging topics.

What You’ll Learn: Patent & FDA Law in Practice
UNDERSTAND the interplay of the PTO and FDA in the patenting of drugs and biologics
LEARN about the approval process for drugs and biologics and their connection to the patent process
COMPREHEND the framework of Hatch-Waxman Paragraph IV litigation and BPCIA patent dance
MAKE SENSE of the relationship between patent and non-patent exclusivity
EXAMINE patent extensions including restorations and adjustments
NAVIGATE the rules and exceptions of the safe harbor
DEVELOP an in-depth and practical knowledge of Hatch-Waxman protocols, including:
- Differences between NDAs, ANDAs, BLAs, aBLAs
- The Orange Book vs. The Purple Book
- 180 Exclusivity
- Bioequivalency
- The Safe Harbor
Who Should Attend
The Hatch-Waxman and BPCIA Proficiency Series is designed for new associates, junior partners, and professionals in the life sciences industry.
Law Firms with Practice Groups
- Life Sciences, Pharmaceuticals and Biopharmaceuticals
- Follow-on Biologics and Biosimilars
- Intellectual Property and Patent Prosecution
- Patent and IP Litigation
- Hatch-Waxman and BPCIA Litigation
- Regulatory and Commercialization
- Trademark & Copyright
- Life Sciences IP Antitrust
Pharma and Biopharmeutical Companies
- C-Suite Executives and Associate Executives
- In-House Counsel (IP, Regulatory, Corporate, Litigation)
- Business Development Executives
Trusted by Leading Organizations
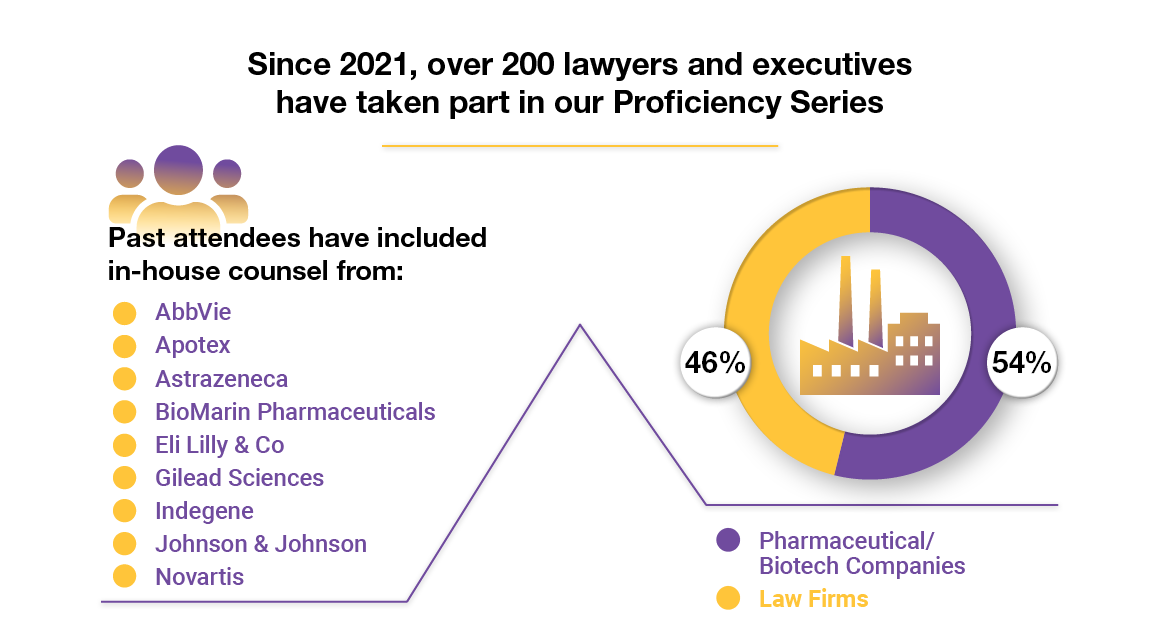
Our course has been taken by professionals from top companies across various industries. Join a growing community of learners from organizations like Novartis, Gilead Sciences, and Sumitomo Pharma who are investing in meaningful professional development.
Participating companies include:
- Abbvie
- AstraZeneca
- Deciphera Pharmaceuticals Inc
- Eli Lilly & Co
- Gilead Sciences Inc
- Indegene
- Johnson & Johnson
- Novartis Institutes of Biomedical Research
- Omers
- Sumitomo Pharma
Essential Education for Life Sciences IP & Regulatory Teams
Comprehensive IP & Regulatory Training
Deepen your understanding of the Hatch-Waxman Act and the Biologics Price Competition and Innovation Act (BPCIA) through a structured, intermediate-level course. Explore critical topics including patent linkage, exclusivities, biosimilars, Paragraph IV litigation, and regulatory strategy for small molecules and biologics.
Expert-Led Instruction
Learn from leading experts in life sciences IP law and regulatory affairs – including experienced attorneys, industry counsel, and former government officials – who will share actionable insights and real-world strategies drawn from high-stakes litigation and policy developments.
Interactive Virtual Experience
Participate in dynamic case studies, practical scenarios, and live Q&A sessions designed to translate complex legal frameworks into practical application for IP counsel, regulatory professionals, and business stakeholders.
Flexible Online Format
Access content in convenient modules designed to fit your schedule. Whether attending live or viewing recorded sessions, you’ll gain lasting value from flexible, high-impact learning.
Continuing Legal and Professional Education
Earn CLE credits and professional development hours applicable to your jurisdiction and organization, while enhancing your expertise in this evolving and highly specialized field.
Accreditation
Accreditation will be sought in those jurisdictions requested by the registrants which have continuing education requirements. This course is identified as nontransitional for the purposes of CLE accreditation.
Learn more“Legal and business professionals who work in the life sciences industry must be well versed in the regulatory components and IP issues that play an integral role in the protection of companies’ products. This immersive series explores a variety of patent and regulatory issues, including essentials and intricacies of Hatch-Waxman and BPCIA litigation and regulation. The Series is so comprehensive, it truly stands apart as the best option to train newer lawyers and in-house counsel.”
Proskauer Rose LLP (2024 Conference Co-Chair)
“Patent litigation continues to generate numerous consequential precedents that impact the strength and scope of patent rights covering pharmaceutical and biopharmaceutical innovations. Further, legislators and regulators continue to increase their oversight of the pharmaceutical and biopharmaceutical industries. This Series covers the essentials of the litigation, regulatory, and business landscape relating to these industries to give attendees a proficiency with the fundamentals of patent prosecution, exclusivities, patent litigation, commercialization, and more. This comprehensive Series is important especially at a time when all attorneys increasingly need to have a holistic and multifaceted understanding of the legal and business aspects of these complex industries.”
Axinn, Veltrop & Harkrider (2024 Conference Co-Chair)
Sponsor Opportunities
With conferences in the United States, Europe, Asia Pacific, and Latin America, the C5 Group of Companies: American Conference Institute, the Canadian Institute, and C5 Group, provides a diverse portfolio of conferences, events and roundtables devoted to providing business intelligence to senior decision makers responding to challenges around the world.

For more information please contact:
Aaron Goldstein
Senior Business Development Manager
Email: [email protected]




